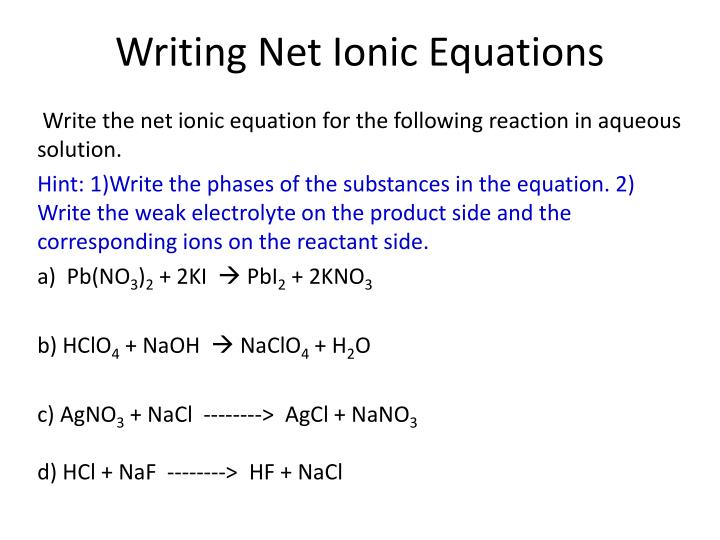
What Does Net Ionic Equation Show. A spectator ion is an ion that does not take part in the chemical reaction and is found in solution both before and after the reaction. Enter an equation of an ionic chemical equation and press the balance button.

Write the balanced molecular equation for the reaction, including the state of each substance. The complete ionic equation indicates all of the dissociated ions in a chemical reaction. The components of this equation include reactants with their physical states, an arrow to show the direction of reaction and the products of reaction with their physical states.
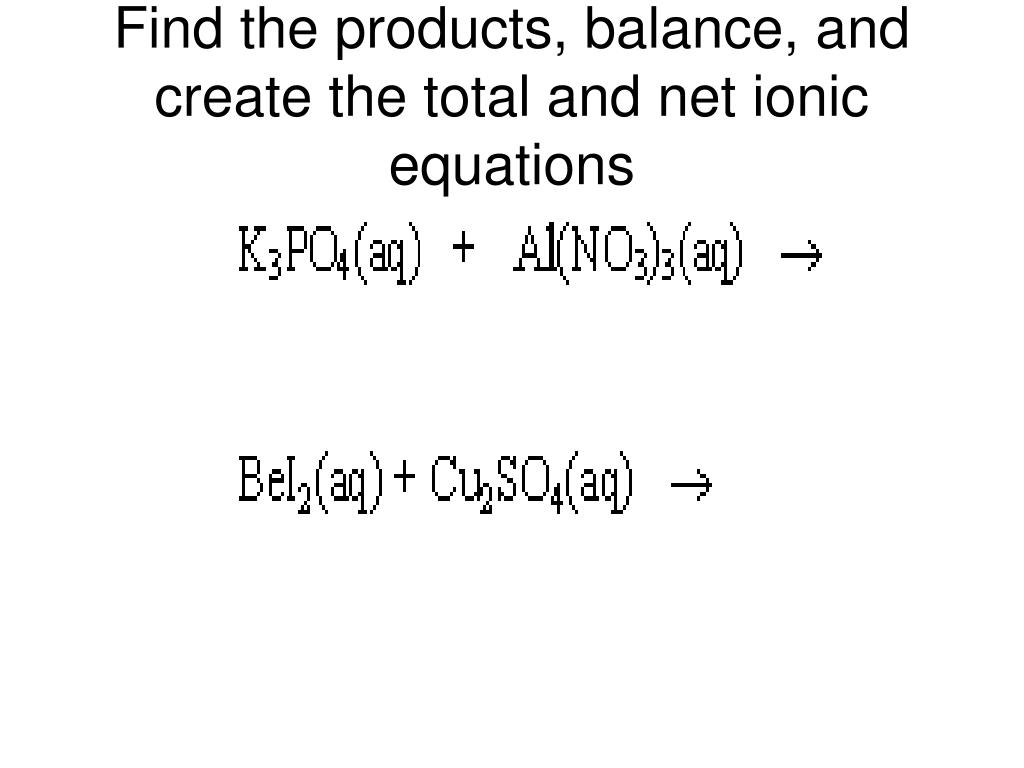 Source: www.chegg.com
Source: www.chegg.com
A molecular equation is useful in describing actual reactant and product substances, it does not tell you what is happening at the level of ions.ca(oh)2 and na2co3 both are soluble ionic substances and therefore strong electrolytes;when they dissolve in water, they go into solution as ions.each foumula unit of. For a double replacement reaction.
To Write The Net Ionic Equation, We’ll Cancel Out All Spectator Ions.
It lists only the substances that are changed. The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially don't participate in the reaction of interest. Ok, notice how we separate each substance with the (aq) designation into ions in our total ionic equation above.
Complete Ionic Equations Show All Ions Present In Solution During A Reaction.
Net ionic equations show only the ions and other substances that change in a chemical reaction. In the net ionic equation, any ions that do not participate in the reaction (called spectator ions) are. Ionic equations show species reacting as their ionic components.
But Firstly, K (Ii) Will Not Form, As Potassium Ionizes To A +1 Charge.
The ions that are canceled out are called spectator ions. We can find the net ionic equation for a given reaction using the following steps: Write net ionic equations for reactions in aqueous solutions.
As A General Rule, If You Balance The Molecular Equation Properly, The Net Ionic Equation Will End Up Being Balanced By Both Mass And Charge.
A net ionic equation, on the other hand, shows only the ions that involve with the reaction. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Writing a net ionic equation step 1:
In The Molecular Equation For A Reaction, All Of The Reactants And Products Are Represented As Neutral Molecules (Even Soluble Ionic Compounds And Strong Acids).
But if the aqueous ions of the reactants equal all the ions of the product, you don’t have a chemical reaction but a mixture. The net ionic equation indicates the chemical species that undergo a chemical change. Click to see full answer.